Abstract
Background: Reports of recurrent genomic alterations in Burkitt lymphoma (BL) have identified multiple recurrent alterations that result in activation of PI3K highlighting the importance of the PI3K/AKT/mTOR pathway in Burkitt lymphomagenesis. In a cell line model of resistant BL, we have previously identified an increase of PI3K/AKT/mTOR pathway activation suggesting a role in therapy resistance. While inhibition of PI3K-delta using the isoform specific inhibitor idelalisib has demonstrated clinical activity in indolent lymphomas, limited single agent activity has been observed in more aggressive variants. Pre-clinical investigation of idelalisib in BL indicated similar somewhat limited in vitro activity with synergistic activity in combination with chemotherapy. Broader inhibition of both upstream PI3K and downstream mTOR may exhibit more significant anti-lymphoma activity.
Objectives: Investigate the in vitro and in vivo activity of the dual pan-PI3K/mTOR inhibitor omipalisib (GSK458) in chemotherapy-sensitive and -resistant BL cell line models.
Methods: Experiments were conducted in Raji, Raji 4RH (chemotherapy-rituximab resistant), Ramos, and Daudi BL cells. Cell viability following exposure to omipalisib +/- chemotherapy was analyzed using Cell-Titer Glo and Alamar blu assays. Induction of apoptosis was assessed by flow cytometry for Annexin V (AV)-propidium iodide (PI) staining. Downstream effects of omipalisib on PI3K/Akt/mTOR signaling were analyzed using western blotting. Cell cycle analysis was performed by flow cytometry using PI staining. Synergy of combination exposures was determined by calculation of the combination index (CI) using CalcuSyn software. In vivo activity was evaluated using disseminated Raji and subcutaneous Ramos SCID mouse xenograft models. The survival end point was hind limb paralysis in the disseminated model and tumor diameter >2cm in the subcutaneous model. Mice were treated with vehicle or omipalisib daily by oral gavage. Median survival was compared by Kaplan-Meier analysis.
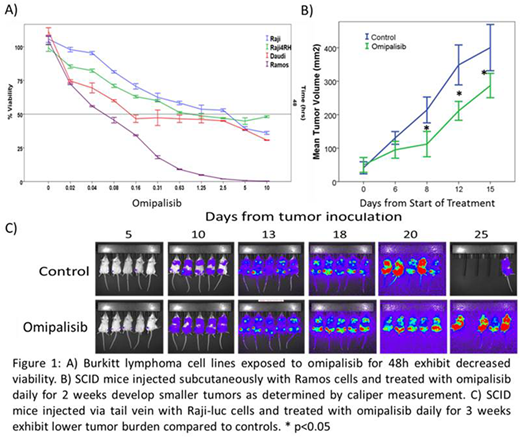
Results: Exposure of BL cells to omipalisib for 24-72 hours resulted in a dose- and time-dependent decrease in viable cells at nM concentrations (48h IC50 values: Raji=1.2uM, Raji 4RH=0.02uM, Ramos=0.01uM, Daudi=0.01uM) (Figure 1A). Marked induction of apoptosis occurred following 72h exposure to omipalisib primarily in chemosensitive cells with half-maximal effect noted at approximately 200nM, but requiring significantly higher concentrations to induce apoptosis in therapy resistant Raji 4RH cells (%AV positive at 200nM: Raji=40.7%, Raji 4RH=4.4%, Ramos=59.4% and Daudi=46.9%). Downstream of PI3K/Akt/mTOR, S6 and GSK3β showed reduced phosphorylation after 30 minute omipalisib exposure. G1 cell cycle arrest occurred in all cell lines following exposure to omipalisib for 72 hours; however, chemotherapy-resistant Raji 4RH cells arrested in G2/M at higher concentrations. BL cells exposed to omipalisib in combination with either doxorubicin or dexamethasone, exhibited synergistic anti-tumor activity (CI<0.9) with synergistic induction of apoptosis in therapy sensitive cells exposed to omipalisib and chemotherapy. NOD-SCID mice injected via tail vein with Raji-luc (provided by Dr. Mitchell Cairo) and treated with omipalisib demonstrated decreased luciferase signal compared to controls (Figure 1C) while mice with established subcutaneous Ramos xenografted tumors treated with omipalisib exhibited slower tumor progression compared to controls (Figure 1B), though with only modest prolongation of survival (median 28 vs 34 days, n=15/group, p<0.05).
Conclusion: Dual PI3K-mTOR inhibitor omipalisib suppresses the PI3K/Akt/mTOR pathway leading to induction of apoptosis, impaired BL cell proliferation in vitro and in vivo and exhibits synergistic in vitro activity when combined with cytotoxic chemotherapy highlighting the relevance of PI3K/Akt/mTOR pathway inhibition as a potential therapeutic option in BL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal